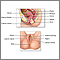
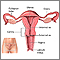
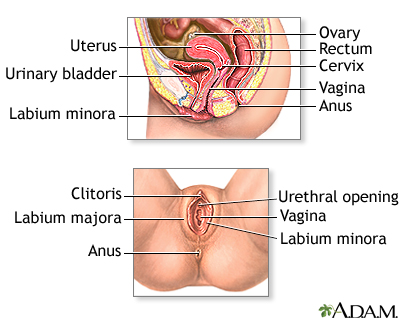
The Pap test mainly checks for changes that may turn into cervical cancer. Cells scraped from the opening of the cervix are examined under a microscope. The cervix is the lower part of the uterus (womb) that opens at the top of the vagina.
This test is sometimes called a Pap "smear" or "cervical cytology."
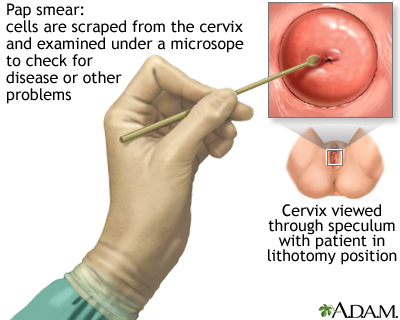
How the Test is Performed
You lie on a table and place your feet in footrests. Your health care provider gently inserts an instrument called a speculum into your vagina to open it slightly. This allows the provider to see your cervix.
Cells are gently collected from your cervix. The sample of cells is sent to a lab for examination.
How to Prepare for the Test
Tell your provider if you:
- Have had an abnormal Pap test or a positive human papillomavirus (HPV) test in the past. HPV is a virus that causes genital warts and cervical cancer.
- Might be pregnant.
DO NOT do the following for 24 hours before the test:
- Douche (douching should never be done)
- Have intercourse
- Use tampons
Try not to schedule your Pap test for when you have your period, but if you are having unexpected bleeding, do not cancel your exam. Your provider will determine if the Pap test can still be done.
For your comfort, you may want to empty your bladder just before the test.
How the Test will Feel
A Pap test causes only mild discomfort for most people. The discomfort it causes is usually similar to menstrual cramps. You may also feel some pressure during the exam.
You may bleed a little bit after the test.
Why the Test is Performed
The Pap test looks for changes in cervical cells that may turn into cervical cancer. Most cervical cancers can be avoided if you have screenings according to the recommended schedule.
Experts disagree about when and how often screening tests for cervical cancer should be done, including Pap tests. Some recommend having Pap tests starting at age 21 and then continuing to have them every 3 years. Others recommend waiting until age 25 and using a primary HPV test (one approved by the FDA for this purpose) to screen for cervical cancer. Discuss this with your provider to choose the best approach for you.
If you are age 30 or over you can do any of the following:
- Continue to have a Pap test every 3 years
- Switch to a test for HPV every 5 years
- Have both the Pap and HPV tests every 5 years
You may not need to have a Pap test if you have had a total hysterectomy (uterus and cervix removed) and have had normal Pap tests in the past.
Most people can stop having Pap tests after age 65 if they've had normal tests in the past. Ask your provider when you should stop screening.
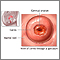
Normal Results
A normal result means there are no abnormal cells present. The Pap test is not 100% accurate, and this is why repeat testing is recommended. Because cervical cancer develops very slowly, repeat Pap tests usually find changes in time for treatment before cancer develops.
What Abnormal Results Mean
The most common abnormal Pap test results are:
Atypical squamous cells of undetermined significance (ASC-US)
- This test result is considered a mild change.
- These changes may be due to an HPV infection, inflammation, or lack of estrogen as occurs in menopause.
- Repeat Pap testing or HPV testing is recommended. If an HPV test is positive, colposcopy is recommended.
Low-grade squamous intraepithelial lesion (LSIL)
- This test result is considered a mild change.
- These changes are often associated with an active HPV infection but may indicate that a precancer or cancer is present.
- Colposcopy is recommended unless an HPV test is negative, in which case repeat testing in one year is often recommended.
Atypical squamous cells, cannot rule out HSIL (ASC-H)
- This test result is considered a relatively severe change.
- These changes may indicate that a precancer or cancer is present.
- Colposcopy is recommended.
High-grade squamous intraepithelial lesion (HSIL)
- This test result is considered a severe change.
- These changes may indicate that a precancer or cancer is present.
- Colposcopy is recommended.
Atypical glandular cells (AGC)
- This test result is considered a severe change.
- These changes may indicate that there is a precancer or cancer of the cells inside the cervix or uterus.
- Colposcopy is recommended and may include a biopsy of the lining of the uterus.
Alternative Names
Papanicolaou test; Pap smear; Cervical cancer screening - Pap test; Cervical intraepithelial neoplasia - Pap; CIN - Pap; Precancerous changes of the cervix - Pap; Cervical cancer - Pap; Squamous intraepithelial lesion - Pap; LSIL - Pap; HSIL - Pap; Low-grade Pap; High-grade Pap; Carcinoma in situ - Pap; CIS - Pap; ASCUS - Pap; Atypical glandular cells - Pap; AGUS - Pap; Atypical squamous cells - Pap; HPV - Pap; Human papilloma virus - Pap cervix - Pap; Colposcopy - Pap; Cervical cytology
References
Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70(5):321-346. PMID: 32729638 pubmed.ncbi.nlm.nih.gov/32729638/.
Newkirk GR. Pap smear and related techniques for cervical cancer screening. In: Fowler GC, ed. Pfenninger and Fowler's Procedures for Primary Care. 4th ed. Philadelphia, PA: Elsevier; 2020:chap 120.
Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131. Erratum in: J Low Genit Tract Dis. 2020;24(4):427. PMID: 32243307 pubmed.ncbi.nlm.nih.gov/32243307/.
Salcedo MP, Phoolcharoen N, Schmeler KM. Intraepithelial neoplasia of the lower genital tract (cervix, vagina, vulva): etiology, screening, diagnosis, management. In: Gershenson DM, Lentz GM, Valea FA, Lobo RA, eds. Comprehensive Gynecology. 8th ed. Philadelphia, PA: Elsevier; 2022:chap 29.
US Preventive Services Task Force website. Cervical cancer: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening. August 21, 2018. Accessed February 6, 2024.
Review Date 1/1/2023
Updated by: John D. Jacobson, MD, Professor Emeritus, Department of Obstetrics and Gynecology, Loma Linda University School of Medicine, Loma Linda, CA. Internal review and update on 02/10/2024 by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team.