

| ||
| August 8, 2005 [posted] | ||
| ClinicalTrials.gov Scope Expanded | ||
|
At present, ClinicalTrials.gov contains nearly 13,900 summaries of clinical research studies in all 50 states and over 100 countries. These studies are sponsored by the U.S. Federal government and industry, as well as non-profit organizations from around the world. Information about registering study information with ClinicalTrials.gov is available at the ClinicalTrials.gov Protocol Registration System (PRS). As reported previously (Update: Expanded ClinicalTrials.gov Search Capabilities. NLM Tech Bull. 2002 Jul-Aug; (327):e5) the number of studies provided by industry sponsors have continued to increase since publication of the Food and Drug Administration's (FDA's) Guidance for Industry (March 2002). The guidance requires Investigational New Drug application (IND) holders to register clinical trials of drug effectiveness for serious or life threatening diseases. In addition, several industry associations, including the U.S.-based Pharmaceutical Research and Manufacturers of America (PhRMA), released the Joint Position on the Disclosure of Clinical Trial Information in January 2005, which encourages registration of industry-sponsored trials at ClinicalTrials.gov.
Scope Expansion Up to October 2004, ClinicalTrials.gov only accepted the submission of trials conducted under FDA INDs or funded by the NIH and other U.S. Federal agencies. To accommodate the ICMJE criteria for reporting critical information about clinical trials at inception, ClinicalTrials.gov expanded its scope to allow registration of all trials that meet two requirements:
The May 2005 ICMJE statement clarified what it means to be "fully registered." This policy statement specified deadline dates by which trials must be registered to be considered for publication. The policy applied to ongoing trials that were not registered at patient recruitment inception as well as trials that started patient recruitment on or after July 1, 2005. Ongoing trials had to be registered before September 13, 2005 to be considered for publication. In addition, the ICMJE adopted a minimal registration data set, developed by the World Health Organization (WHO) technical advisory group, required to be provided and displayed by an acceptable clinical trial registry. The NLM responded to the May 2005 ICMJE statement in two ways.
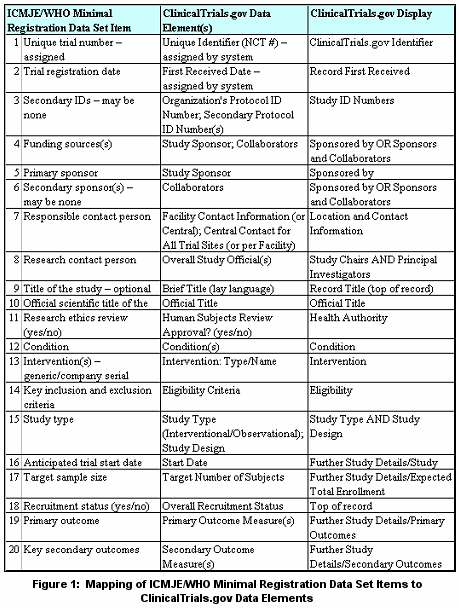
While ClinicalTrials.gov provides a mechanism for sponsors to submit information required by ICMJE, whether the content is sufficient to satisfy the criteria may only be determined by the ICMJE, or biomedical journal editors who choose to follow the ICMJE policies. Information about the following topics is available at ClinicalTrials.gov Protocol Registration System (PRS):
Contact Information
By Anna Ripple, Tony Tse, PhD, Deborah A. Zarin, MD Ripple A, Tse T, Zarin DA. ClinicalTrials.gov Scope Expanded. NLM Tech Bull. 2005 Jul-Aug;(345):e6. | ||

 linicalTrials.gov
linicalTrials.gov