New Look for ClinicalTrials.gov
[Editor's Note: The new ClinicalTrials.gov user interface was launched on November 8, 2007 at //ClinicalTrials.gov/.]in September 2007, ClinicalTrials.gov will launch a new user interface, which represents the first significant change to the interface since February 2000. The new user interface will run in parallel with the previous version and after appropriate testing, the new user interface will permanently replace the previous version (approximately two to four weeks after launch). This article describes the new features, including highlighted search terms, more display options, and additional capabilities for viewing studies and search results by topic. Improvements were also made to "Advanced Search" and the display by location on the world map.
ClinicalTrials.gov is the NLM®-developed Web-based registry of clinical research studies. It provides patients, clinicians, researchers, and other members of the general public with access to information about interventional and observational studies including clinical trials of drugs, devices and other interventions. As of August 2007, ClinicalTrials.gov contained over 44,000 clinical research studies in all fifty states and in 150 countries.
As reported previously, the scope of ClinicalTrials.gov was expanded to accommodate the International Committee of Medical Journal Editors (ICMJE) policy requiring trial registration for publication of research results (NLM Tech Bull. 2005 Jul-Aug;(345):e6). Note that the ICMJE issued an updated statement on trial registration [this link was removed because is it was no longer valid] in June 2007 expanding the requirements to "any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes."
New Features
User Interface Overview
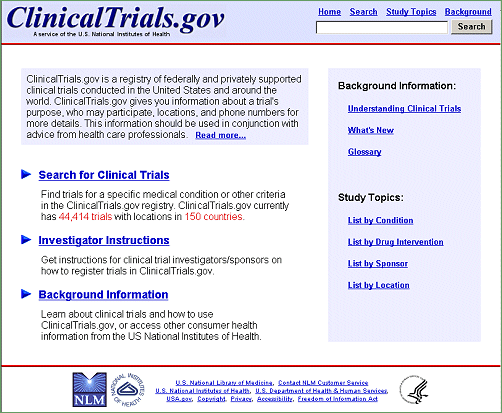
While the new interface enhances site functionality and navigation significantly, the core aspects of ClinicalTrials.gov have been preserved. The homepage (see Figure 1) now provides explicit links for searching the database (Search for Clinical Trials) and for investigators to register studies (Investigator Instructions). A search box with four navigation links at the top right of each page has replaced the menu bar, allowing new searches for studies to be launched at any time. Pages are now organized by context-sensitive tabs — that is, their labels and functions change as a search progresses from (1) submitting search terms to (2) viewing a list of results to (3) reviewing summaries of individual studies (described below). New features provide users with different options to display results lists and study information based on their needs.
Search Term Highlighting
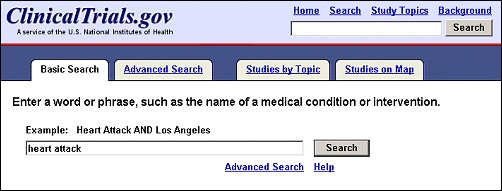
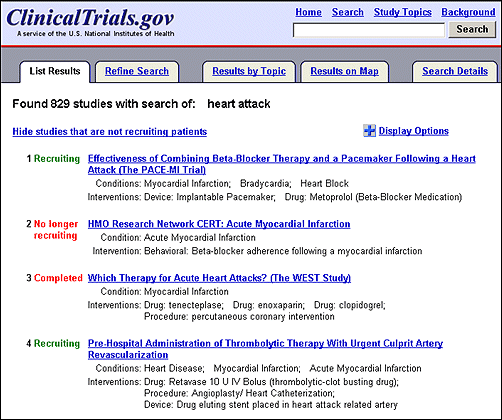
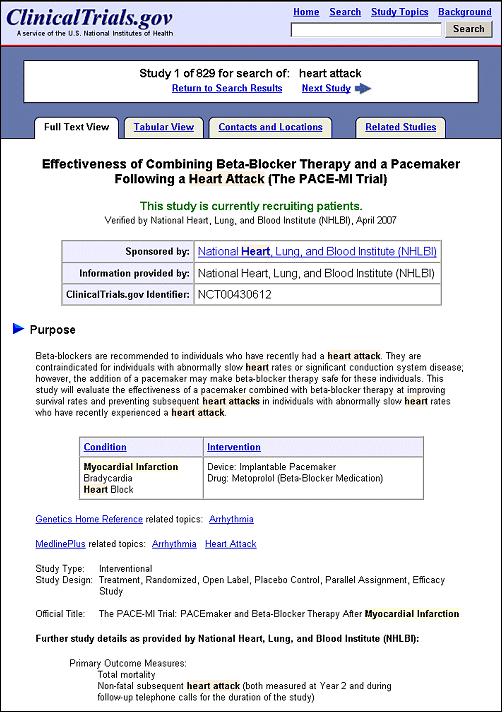
Search terms and synonyms are now highlighted and bolded in study records. A basic search conducted on heart attack retrieved 829 studies, open and closed to recruitment (see Figures 2a and Figures 2b). Click on the title of a study record on the List Results page to review the details of a study. In the first retrieved record, both heart attack and its related term myocardial infarction are highlighted (see Figure 3). The search terms heart OR attack are highlighted in a different color than the related term myocardial infarction. For additional information on search capabilities of ClinicalTrials.gov, see previous publications Update: Expanded ClinicalTrials.gov Search Capabilities. NLM Tech Bull. 2002 Jul-Aug; (327):e5. and Searching ClinicalTrials.gov. NLM Tech Bull. 2000 Sep-Oct;(316)e1.
Display Options
- List Results Page
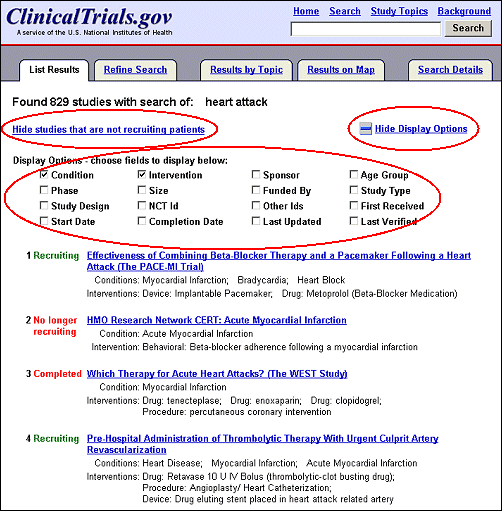
The default display on the List Results page includes condition(s) and intervention(s) being studied (see Figure 2b). Click on "Display Options" ("+" icon or link), above the first study listing in the upper right-hand corner, to reveal sixteen data elements that may be displayed (see Figure 4) in addition to the study title, recruiting status, conditions and interventions. As an option is selected, the corresponding information appears underneath each study listing. After selecting the desired options click on "Hide Display Options" ("-" icon or link) in the upper right-hand corner (see Figure 4) to hide the menu while saving selections for the duration of the search session.
The List Results page defaults to displaying all studies, independent of recruiting status (e.g., recruiting, no longer recruiting, completed). To limit the list to display only studies recruiting participants, click on the "Hide studies that are not recruiting patients" link (left side of the page, directly above the first study listing (see Figure 4).
- Study Record
The "Tabular View" and "Contacts and Locations" tabs provide two new display options for viewing a study record. The default (labeled "Full Text View") is similar to the study page on the previous version of the ClinicalTrials.gov site (see Figure 3). Click on the "Tabular View" tab to see a tabular list of data elements, except for contact and location information (see Figure 5). Click on the "Contacts and Locations" tab to view detailed contact information and study locations (see Figure 6).
Viewing Studies by Topic/Category
- Studies by Topic and Study Topics
"Studies by Topic" and "Study Topics" expand on the previous version's feature "Listings" and allows users to view all studies by four main categories: Conditions, Drug Interventions (new), Sponsors, and Locations. Access categorical information for all studies registered in ClinicalTrials.gov by several methods: select "Study Topics" above the search box on the homepage, select a study topic from the right hand side bar on the homepage, or select the "Studies by Topic" tab on the Basic Search page. Within each main category, studies are grouped alphabetically or by "subcategories." An example of viewing studies by category is explained in the following section.
- Results by Topic
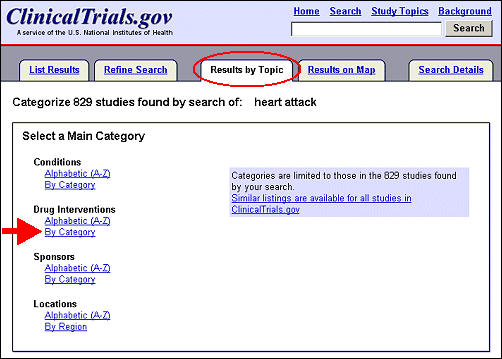
"Results by Topic" is similar to "Studies by Topic" and "Study Topics," but accessed by tabs on the List Results page after a query has been submitted. For example, a Basic Search for heart attack yielded 829 studies (see Figure 2b). Select the "Results by Topic" tab at the top of the List Results page (see Figure 7).
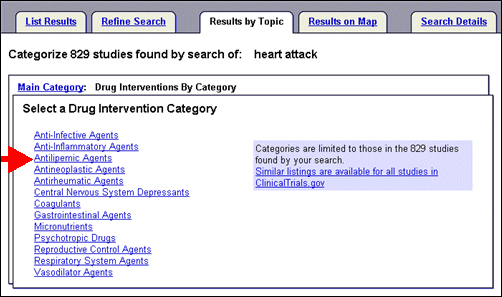
Select a main category and method of viewing (alphabetic or by category) such as "Drug Interventions By Category" (see Figure 8).
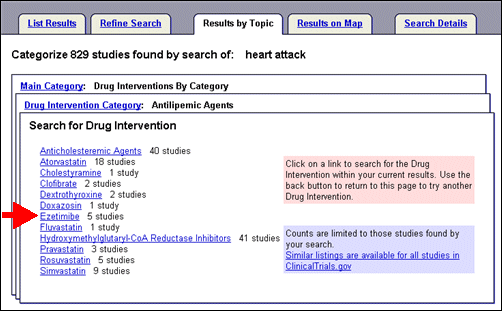
Select "Antilipemic Agents," from the list of drug intervention categories to view types and names of drugs classified as "Antilipemic Agents" (see Figure 9) that are part of study records within the search of heart attack.
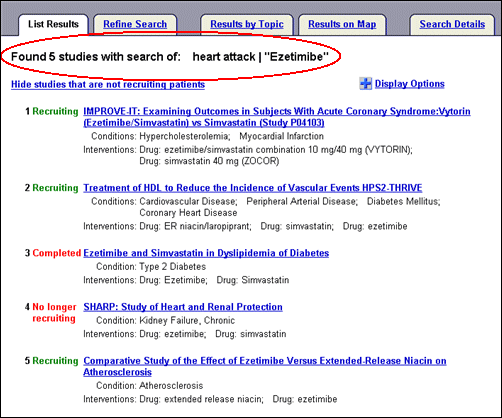
Select "Ezetimibe" from the drug interventions provided to display a new List Results page (see Figure 10) with a clearly marked search trail at the top of the page: "Found 5 studies with search of: heart attack|"Ezetimibe" (i.e., equivalent to the query: heart attack AND Ezetimibe).
Additional Features
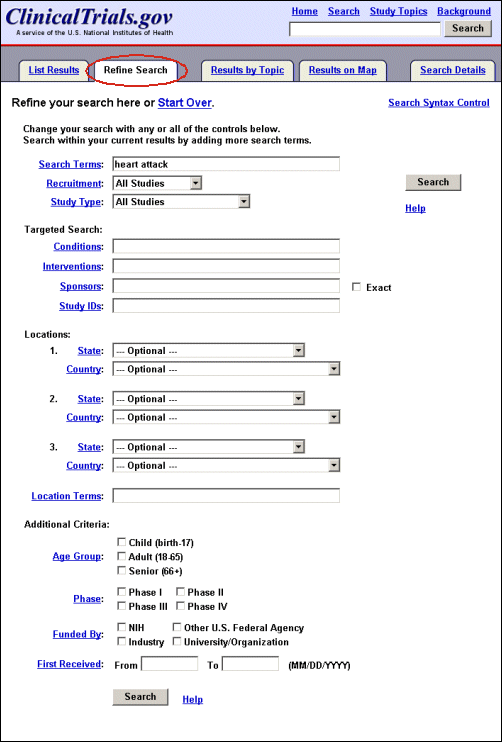
"Advanced Search" (previously "Focused Search") is now accessible by a tab or a link on the Basic Search page (see Figure 2a). Advanced Search includes new items, while retaining many of the features described previously (Update: Expanded ClinicalTrials.gov Search Capabilties. NLM Tech Bull. 2002 Jul-Aug; (327):e5). Users can now limit search based on recruitment status (open or closed to enrollment), study type (interventional, observational, expanded access), and date study record was first received by ClinicalTrials.gov. In addition, users can search for a specific study sponsor and search multiple locations at once (e.g., Washington DC OR Virginia OR Maryland). The function of Advanced Search can also be applied to a search result by selecting the "Refine Search" tab (previously "Search Within Results") on the List Results page and the user’s original search term(s) will be retained (see Figure 11).
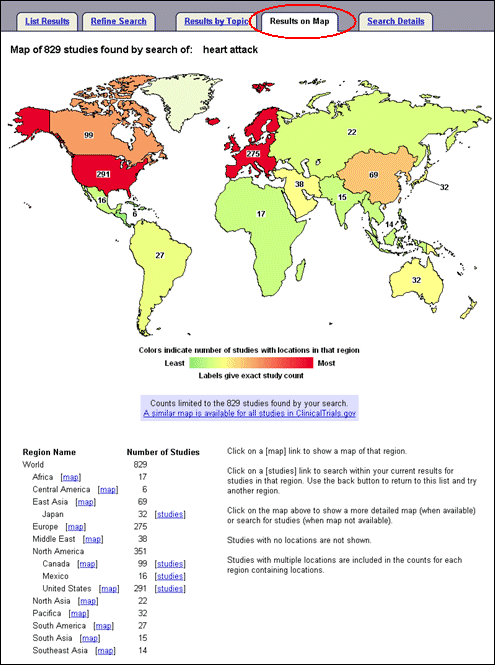
Users can navigate to the next or previous study record from the top of the study record without having to return to the List Results page. The map of study locations is enhanced and users can view the number of registered studies in different regions of the world for a specific search (see Figure 12) or for all studies in the ClinicalTrials.gov database.
Contact Information
Please send your comments or questions to: custserv@nlm.nih.gov
Williams RJ, Tse T. New Look for ClinicalTrials.gov. NLM Tech Bull. 2007 Sep-Oct; (358):e2.