 he fundamental search features in ClinicalTrials.gov,
the National Library of Medicine (NLM)-developed, Web-based information resource for patients, family members,
and members of the public, were described previously (NLM Tech Bull. 2000
Sep-Oct; (316):e1) and are summarized below in the Background section. This article describes the new capabilities, added
in May 2002, to help refine searching. The Help page also provides information on these features.
he fundamental search features in ClinicalTrials.gov,
the National Library of Medicine (NLM)-developed, Web-based information resource for patients, family members,
and members of the public, were described previously (NLM Tech Bull. 2000
Sep-Oct; (316):e1) and are summarized below in the Background section. This article describes the new capabilities, added
in May 2002, to help refine searching. The Help page also provides information on these features.
At present, ClinicalTrials.gov contains more than
6,500 clinical studies in approximately 69,000 locations worldwide sponsored by the Federal government,
the pharmaceutical industry, and private non-profit sources.
Please see the About page for up-to-date
information and statistics.
The number of clinical trials in the system has substantially increased since the finalization
of the Food and Drug Administration's (FDA's) Guidance for
Industry: Information Program on ClinicalTrials.gov for Serious or Life-
Threatening Disease and Conditions. The guidance document requires all IND (Investigational New Drug)
holders to register clinical trials for serious or life threatening diseases with ClinicalTrials.gov.
BACKGROUND
The U.S. National Institutes of Health (NIH) developed ClinicalTrials.gov, through the NLM, to
provide patients, family members, health care professionals, and members of the public easy access to
information on clinical trials for a wide range of diseases and conditions. The Web site can be accessed
at http://clinicaltrials.gov/
Finding Studies
Three mechanisms are available for finding clinical study
protocol records - Basic Search, Focused Search, and Browse:
- Basic Search (or Search), which is provided on the Home and Search pages, allows users to search broadly.
- Focused Search, accessible through a link under Search by Specific Information header on the Home Page, allows users to narrow searches by specific criteria, such as Disease, Location, Treatment, and Sponsor.
- Browse allows you to review clinical studies by selecting from successive groupings: alphabetically by disease name, by disease headings, or by sponsor type.
Synonym Expansion
When using either the Basic or Focused Search, the system automatically includes words or phrases that are synonymous with the search terms. For example, a search for "heart
attack" will also include a search for "myocardial infarction." Plurals for search terms are also included.
Spell Checking
Alternative words may be offered if a search term is not found in the database. For example, the query "amaloid" produces
two alternatives: "amyloid" and "alkaloid." Clicking on the circle to the left of a word automatically reruns the search
with word substitution.
Viewing the Results
On the Search Results page, the query used to obtain these results is shown at the top of the page. Underneath,
retrieved studies are listed in a brief format, including:
- recruitment status
- study title
- condition(s) being studied
Status indicates whether or not a trial is recruiting patients. "Not yet recruiting" and "Recruiting" are shown in
green; "No longer recruiting," "Completed," "Suspended," and "Terminated" are displayed in red. Trials
with these red status indicators are not automatically retrieved when
searching; select "Show all trials, including those no longer recruiting patients" to include these. Titles are hotlinks; clicking on them retrieves the complete record
for the clinical study. "Conditions" indicates topics of study.
To view multiple studies, click on the checkboxes of the studies you wish to view and click on the "Display
Selected Studies" button at the bottom of the page.
UPDATE
Search Within Results
Users often wish to modify or limit their results after an initial
search. For example, after determining that there are many
stage 2 breast cancer studies, a user may wish to narrow the results to
those studies located or active in Mississippi.
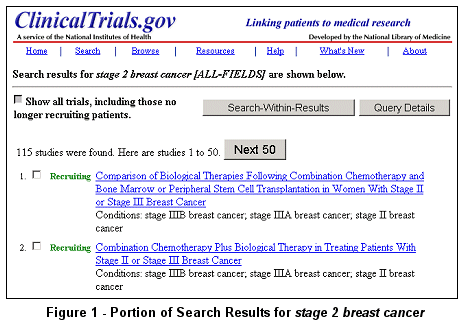
A Basic Search for studies on stage 2 breast cancer
currently yields more than 100 studies (see Figure 1).
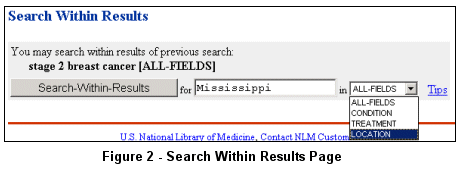
Use the "Search-Within-Results" button at the top of the Search Results page to limit
the retrieval set to studies that have participating facilities in Mississippi (see Figure 2).
Click on the "Tips" link on the Search Within Results page for brief
definitions of the fields that may be used to limit a search (see Figure 3).

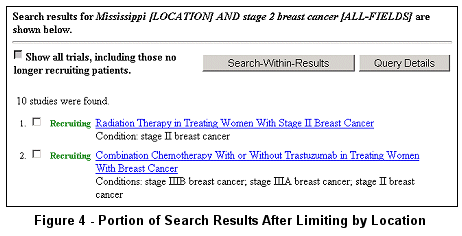
The modified search of stage 2 breast cancer limited to studies having at least one
location in Mississippi returns about 10 studies (see Figure 4).
Search Operators
ClinicalTrials.gov supports the use of Boolean Operators, Phrase Searching, and Field Specification in all search text
boxes: Basic Search, Focused Search, Search-Within-Results, and
Modify Your Search (under Query Details).
- Boolean Operators
Boolean queries may be constructed using any of the following:
All Boolean operators need to be upper-case letters. (Angle brackets, previously required, should not be used.)
Parentheses may be used to nest clauses.
Examples:
- leukemia AND chemotherapy
- (aspirin OR ibuprofen) AND heart disease
- immunodeficiency NOT AIDS
- Phrase Searching
Quotes limit search to a specific phrase.
Example: acute HIV infection
The quoted query term "acute HIV infection" will
retrieve studies containing the phrase acute HIV infection (and
recognized synonyms). These synonyms come from the Unified Medical Language System.
When no quotes are used, ClinicalTrials.gov searches
for both the phrase and "relaxed" forms of the phrase (and recognized
synonyms). Relaxed forms are the intersection (ANDing) of all combinations of words found in the original query, while
maintaining word order in the search strategy.
The unquoted query, acute HIV infection, will
retrieve studies that contain the following:
- acute HIV infection
- acute HIV AND infection
- acute AND HIV infection
- acute AND HIV AND infection
| Query |
Search Strategy Equivalence |
| "acute HIV infection" |
acute HIV infection |
| acute HIV infection |
acute HIV infection OR
(acute HIV AND infection) OR (acute AND HIV infection) OR (acute AND HIV AND infection) |
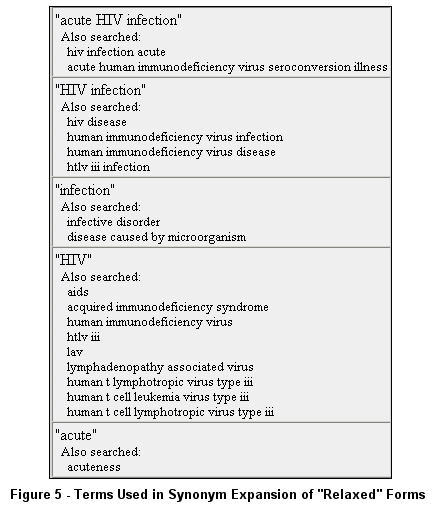
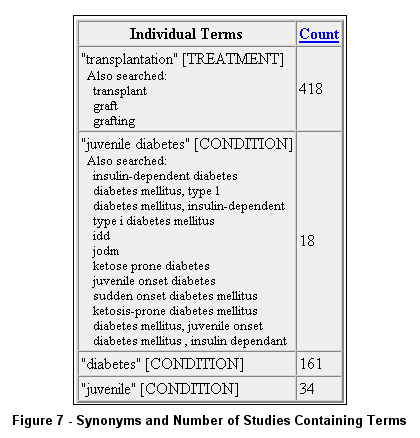
Each "relaxed" form of the above query (no quotes) includes synonym expansion using terms shown in Figure 5 as found in Query Details.
Studies found by the relaxed forms are ranked on the Search Results page according to degree of relaxation: "more relaxed" forms (more ANDs) are ranked lower than those forms closer to the original phrase.
The relaxation strategy is the underlying mechanism for generating Query Suggestions (see "Alternative Search Formulations for Natural Language Searches" discussed later in the article).
- Field Specification
Field specification is used to specify the part of the study to be searched. These fields correspond to the limits in Search-Within-Results. All characters need to be upper case letters. Each field specified must be enclosed in square brackets.
| Field Operator |
Search Strategy Equivalence |
| [ALL-FIELDS] |
Basic Search; Focused Search: Additional Terms |
| [CONDITION] |
Focused Search: Disease or Condition |
| [TREATMENT] |
Focused Search: Experimental Treatment |
| [LOCATION] |
Focused Search: Trial Location |
Example:
The search juvenile diabetes [CONDITION] NOT transplantation [TREATMENT]
retrieves studies using therapies other than transplantation for treating juvenile diabetes.
Query Details: Modify Your Search and Query Suggestions
Users sometimes wish to reformulate their search strategies and/or see terms used after synonym expansion and "relaxed" forms.
After submitting a search, use the Query Details button at the top of the Search Results page to edit a search
or to view synonyms used (see Figure 6 and Figure 7).

Alternative Search Formulations for Natural Language Searches
Some end-users use natural language searches. To assist them in reformulating their searches, ClinicalTrials.gov provides suggestions for alternative
combinations of search terms based on the initial search. For example, the query does aspirin treat heart disease? results in no retrieval. However,
different combinations of search terms are suggested (see Figure 8).
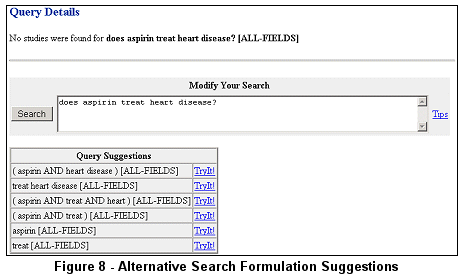
Clicking on TryIt! performs the associated search.
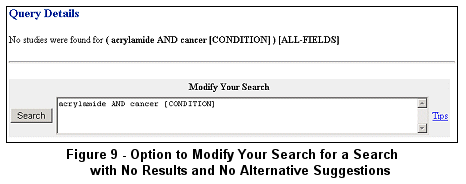
If no studies are found after submitting a search and no alternative search term combinations are offered, the Query
Details page is returned with an option to modify the search (see Figure 9).
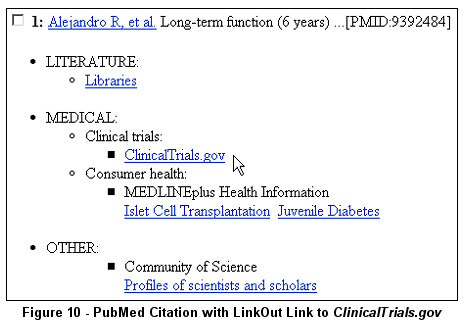
PubMed Citations linked to ClinicalTrials.gov studies via LinkOut
For ClinicalTrials.gov study records that have links to PubMed citations, there
are now reciprocal links back to the ClinicalTrials.gov study records from the PubMed
citations via the LinkOut display (see Figure 10).
Links to Search Requests
A syntax is available for creating "canned searches" in ClinicalTrials.gov. Librarians, Web developers and other information professionals may find this mechanism useful for directing end-users to particular sets of study records through single links. For example, a listing of studies limited to specific diseases, particular locales, or a sponsor may be useful. The HTML for the link combines the command line operators described previously with standard URL (uniform resource locator) encoding.
Example:
Stage 2 breast cancer studies at ClinicalTrials.gov
Such live "query links" to ClinicalTrials.gov are superior to downloading and storing files locally because it ensures that end-users will receive the most current and accurate information.
- Simple Queries
The syntax for performing simple queries, searches involving a single field and term, can be easily generated using URLs. For additional information, see the Linking page.
Examples of URLs for simple queries:
URLs may also be used to link to specific studies by referencing the NLM-assigned unique NCT number: http://clinicaltrials.gov/show/NCT00004451.
ClinicalTrials.gov Web pages may also be linked.
Example: http://clinicaltrials.gov/info/resources links to the Resources page,
which provides background information and general resources about clinical trials.
- Complex Queries
The syntax for performing complex queries requires familiarity with the search operators described previously and standard URL encoding. Any search that can be created at the ClinicalTrials.gov interface (e.g., Basic and Focused Search) may be encoded in a single link, using the template:
http://clinicaltrials.gov/search/term={URL-ENCODED QUERY}
A left- and right-square bracket must enclose specific fields to be searched. The URL encoding for these
characters is listed, in addition to the URL encoding for double quotes which can be used to limit
a search to a specific phrase. Any spaces in the URL are encoded with the plus sign "+".
| Character |
Description |
URL Encoding |
| [ |
left square bracket |
%5B |
| ] |
right square bracket |
%5D |
| " |
double quote |
%22 |
Source: World Wide Consortium - http://www.w3.org/
Example:
To encode the query juvenile diabetes [CONDITION] NOT transplantation [TREATMENT]
as a link in a Web document:
| Original Query |
juvenile diabetes [CONDITION] NOT transplantation [TREATMENT] |
| URL Encoded |
(juvenile+diabetes)+%5B
+[CONDITION]+%5D+NOT+transplantation
+%5B+[TREATMENT]+%5D |
When encoded in HTML:
http://clinicaltrials.gov/search/term=
(juvenile+diabetes)+%5B+CONDITION+
%5D+NOT+transplantation+%5B+TREATMENT+%5D
Contact Information
Please send your comments or questions to custserv@nlm.nih.gov.
By Tony Tse,
Sue O. Johnson,
and
Anna Ripple
Lister Hill National Center for Biomedical Communications

Tse T, Johnson SO, Ripple A. Update: Expanded ClinicalTrials.gov Search Capabilities. NLM Tech Bull. 2002 Jul-Aug;(327):e5.
| 











 he fundamental search features in
he fundamental search features in 