



Table of Contents: 2012 JULY–AUGUST No. 387
Pash J. A New System of Registry Number Identifiers for Chemicals in the MeSH Database. NLM Tech Bull. 2012 Jul-Aug;(387):e7.
Food and Drug Administration (FDA) Substance Registration System (SRS) - Unique Ingredient Identifiers (UNIIs) are being introduced into the Medical Subject Headings (MeSH) vocabulary starting with the 2013 MeSH Supplementary Concept Records (SCRs). The UNIIs are an integral part of the FDA Substance Registration System. They appear in several databases such as the Veterans Administration National Drug File Reference Terminology, the USP Dictionary of United States Adopted Names (USAN) and International Drug Names (INN), and the RxNorm database. Each UNII is a unique series of ten characters that includes a check digit to ensure data integrity (see Figure 1).
The UNIIs will occur in the RN (Registry Number) field of MeSH Supplementary Concept Records (SCRs) for chemicals with structures, and serve as a new system of unique identifiers that will enhance existing Chemical Abstracts Service (CAS) registry numbers and Enzyme Commission (EC) numbers. An SCR contains one or more concepts that each allow only one RN number occurrence. Therefore, the UNII insertion process will retain any replaced CAS registry number or EC number by moving it to Related Number (RR) fields in the MeSH record. Note that in PubMed, any RN or RR value can be searched (either with no search tag or with the [rn] search tag) to retrieve the concept; all RR occurrences map to the RN value of the record. Therefore, moving CAS registry or EC numbers to RR fields should not affect existing searches using these values. Terms in MeSH that match the FDA Substance Registration System will receive an additional Thesaurus ID of FDA SRS (20xx), where (20xx) is the year the UNII for that term was first associated with the MeSH concept. This initial update will affect approximately 8,000 SCR records for the 2013 MeSH year.
For further information about the FDA Substance Registration System – UNIIs see: //www.fda.gov/ForIndustry/DataStandards/SubstanceRegistrationSystem-UniqueIngredientIdentifierUNII/default.htm
FDA Substance Registration System
Preferred Substance Name: DEFLAZACORT
UNII: KR5YZ6AE4B
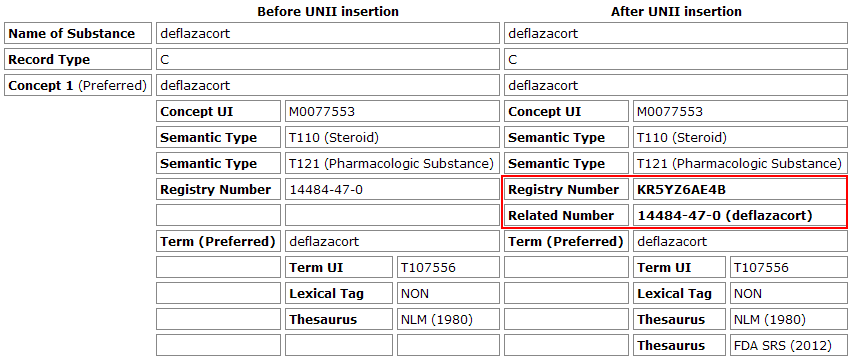
After the UNII insertion as the RN, note in Figure 1 that the new Related Number (RR) is the original Registry Number (RN) with the preferred term appended to it. Because MeSH allows multiple RR numbers in a record, the appended name will maintain the identity of each RR. This Number (name) combined format is a previously established format for the RR field, so the use of it for this purpose should not interfere with systems that use MeSH.
By
James Pash
MeSH Section