



Table of Contents: 2017 NOVEMBER–DECEMBER No. 419
Wolf K, Yu A, Ide N, Williams RJ, Tse T. ClinicalTrials.gov: Further Enhancements to Functionality. NLM Tech Bull. 2017 Nov-Dec;(419):e9.
On December 18, 2017, the National Library of Medicine (NLM) released a new set of updates to ClinicalTrials.gov as part of its ongoing effort to enhance the usability of the database (see ClinicalTrials.gov: First in a Series of Changes to Improve Usability for Stakeholders). Most of the features provided in the current release were previously available for public testing on the beta site starting in November (see New ClinicalTrials.gov Beta Version Available for Public Testing). This article highlights newly added key features, which were informed by research with end-users representing various stakeholder groups and the NLM partnership with 18F, a federal government digital services consultancy. Information about future changes to ClinicalTrials.gov will be provided on a new What's New page.
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world operated by NLM. The database contains information on over 261,000 clinical studies and expanded access (or "compassionate use") to investigational new drugs. Summary results entries for over 29,000 of these studies are also posted in a tabular format on ClinicalTrials.gov. Information listed on ClinicalTrials.gov is provided and updated by the study sponsor or investigator, and listing does not mean it has been evaluated by the U.S. Federal Government.
Updated Search on Homepage
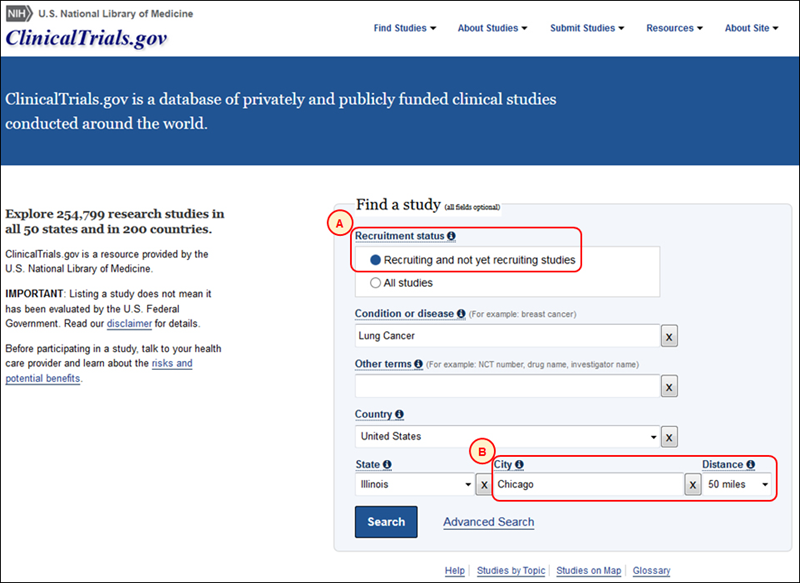
The updated homepage includes a new way for users to limit their searches to studies that are currently recruiting or will be recruiting participants (see A in Figure 1). Additionally, users can now search for studies located within a specified distance from a city (see B in Figure 1, which shows a query for recruiting or not yet recruiting studies for lung cancer within a 50-mile radius of Chicago, Illinois). Together, these new features will help users find information about potential studies in which to participate.
Updated Modify Search on Results Page
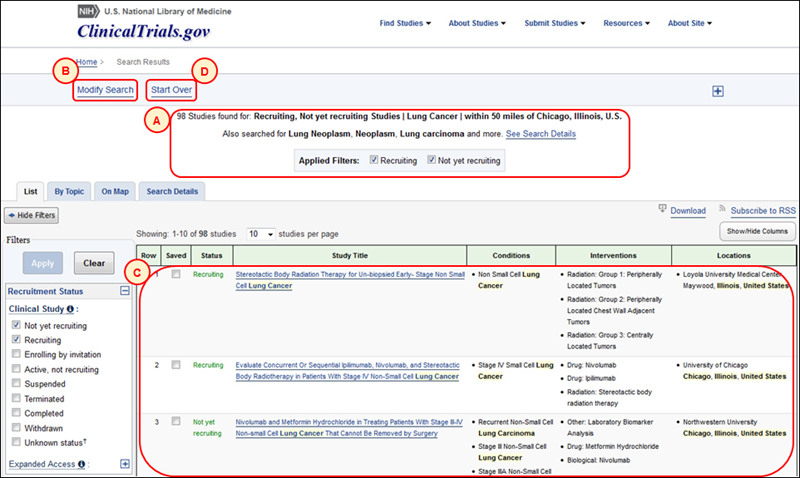
After a query is submitted, the Search Results page displays the number of studies found in ClinicalTrials.gov, the user-entered query terms and filters, and related terms that are automatically searched (see A in Figure 2, which shows synonyms for "lung cancer"). The Modify Search link (see B in Figure 2) allows users to refine the current search directly on the Search Results page. Note that the Modify Search fields (see B in Figure 3) are initially hidden to ensure that the list of study records found is visible on the screen (see C in Figure 2). The Start Over link (see D in Figure 2) returns users to the homepage to conduct a new search.
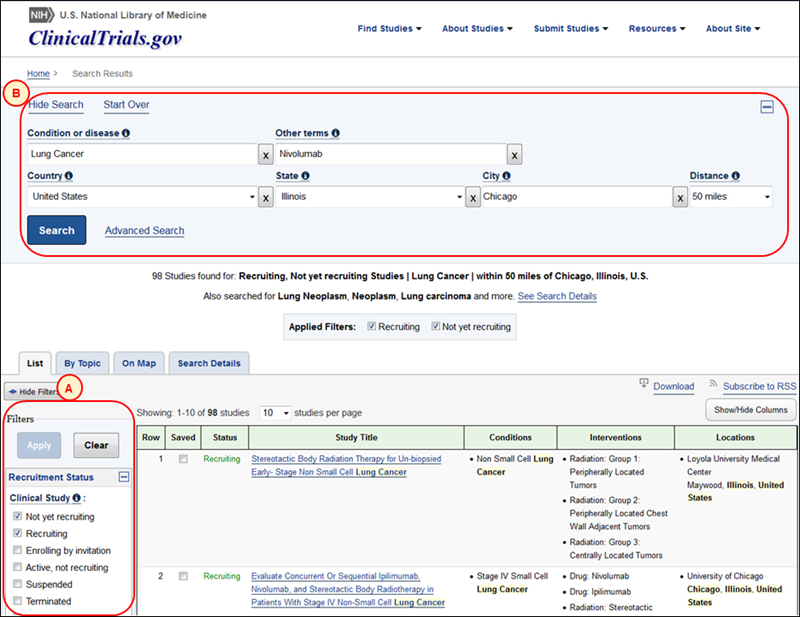
To refine a search, use Filters to add or remove limits (see A in Figure 3) and Modify Search to display and update the search fields and terms used in the current query (see B in Figure 3, in which a drug name, "Nivolumab," has been added in the Other terms field to narrow the search query shown in Figure 2).
In-Context Glossary Display
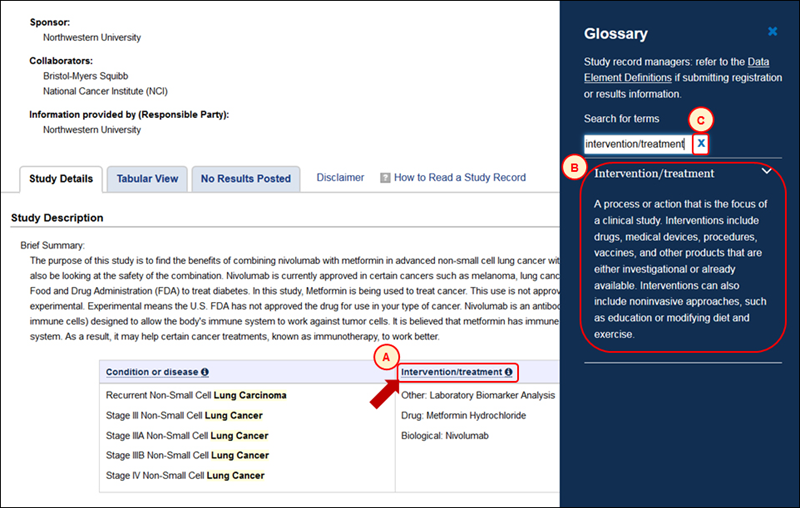
The new glossary feature allows users to look up definitions of terms used on ClinicalTrials.gov while continuing to view the page on which a term appears (i.e., in context). Terms linked to glossary entries are identified by an information icon ("i" in a blue circle) throughout the ClinicalTrials.gov site (see A in Figure 4). Clicking on a term opens a glossary panel from the right side of the screen to display the description for that term (see B in Figure 4, which shows the glossary entry for Intervention/treatment, which appears on the study record at A). The search box at the top of the glossary panel allows users to find entries for other terms. Note that clicking the "x" next to the glossary search box (see C in Figure 4) clears the box and reveals a full list of glossary entries. This glossary feature will help users understand words and phrases frequently used on ClinicalTrials.gov. (Sponsors and investigators should refer to the ClinicalTrials.gov Data Element Definitions documents for help with the data items required during registration and results submission.)
Results Submitted Tab
Information submitted by a study sponsor or investigator undergoes a quality control (QC) review process before being displayed on ClinicalTrials.gov. NLM staff members review all submissions for apparent errors, deficiencies, or inconsistencies. If "major issues" are identified by ClinicalTrials.gov during QC review, the submission is returned to the study sponsor or investigator with comments. Submitted information is publicly displayed (i.e., posted) after all major issues have been corrected or addressed.
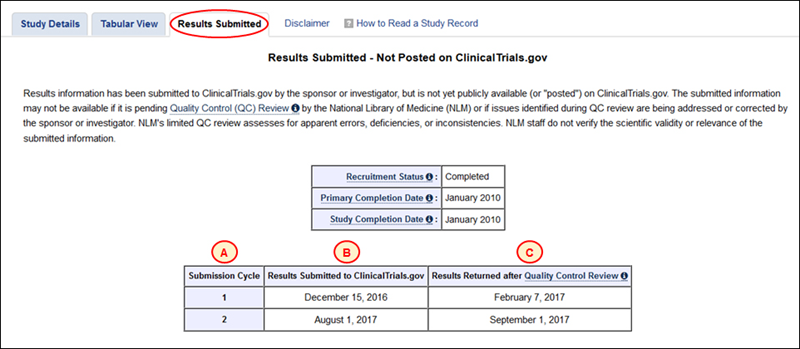
The new Results Submitted tab displays a table that helps users track the QC review status for submitted results information that are not yet posted on ClinicalTrials.gov. After a study sponsor or investigator initially submits results information, the No Results Posted tab on a study record is updated to Results Submitted" (see Figure 5).
Clicking on this tab displays a table of dates. Each submission of results information prior to first posting is shown in a table row that is identified by sequential numbers in the Submission Cycle column (see A in Figure 5). The date on which results information is submitted to ClinicalTrials.gov within each cycle is listed in the second column (see B in Figure 5). If at least one major issue is identified during QC review in a submission cycle, the date on which NLM returns results information to a study sponsor or investigator with QC review comments is listed in the third column (see C in Figure 5). For example, Figure 5 shows that study results were first submitted to ClinicalTrials.gov on December 15, 2016 and returned to the study sponsor or investigator after QC review on February 7, 2017. Overall, results information has been submitted two times without posting (i.e., 2 submission cycles), with the last event being the return of QC review comments from NLM to the sponsor or investigator on September 1, 2017. After all identified major issues have been corrected or addressed by a study sponsor or investigator and submitted, the results information will be posted as part of the study record on ClinicalTrials.gov and the tab label will be changed to "Study Results."
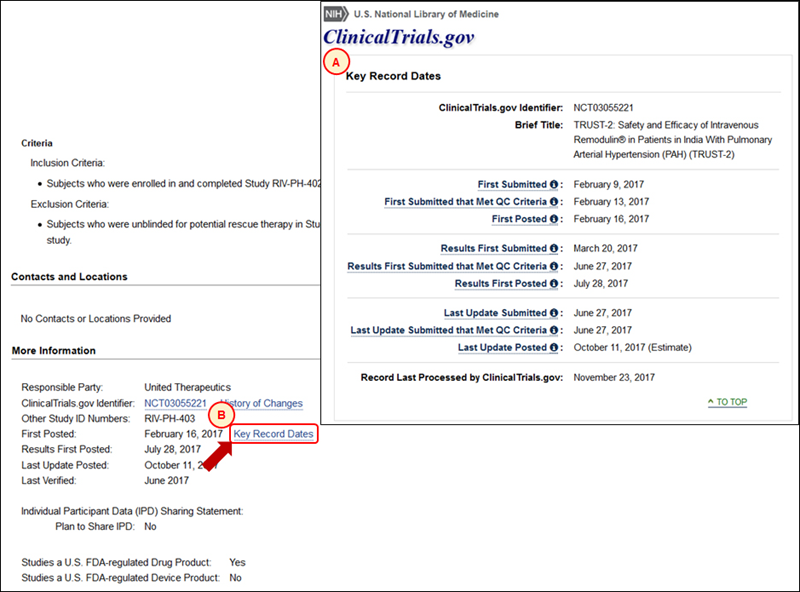
Key Record Dates
A listing of key dates for record-related milestones (see A in Figure 6) is accessible by clicking the Key Record Dates link at the bottom of a study record (see B in Figure 6). The Key Record Dates page allows users to identify when registration and, if applicable, results information was first submitted to ClinicalTrials.gov by the sponsor or investigator and first posted on ClinicalTrials.gov, as well as the date of the first submission that met QC criteria. It also lists when the last update was submitted and posted, and the date of submission for the last update that met QC criteria.
Note that when the QC review process for submitted information requires only a single submission cycle (i.e., no major issues are identified after initial submission), the dates displayed for "submitted that met QC criteria" and "submitted" will be the same (e.g., Last Update Submitted and Last Update Submitted that Met QC Criteria are both listed as "June 27, 2017" in Figure 6). In contrast, when the QC review process takes two or more submission cycles, the date displayed for "submitted that met QC criteria" will be later than the date for "submitted" (e.g., Results First Submitted is "March 20, 2017" while Results First Submitted that Met QC Criteria is "June 27,2017"). For descriptions of each key record date, see Glossary of Common Site Terms.
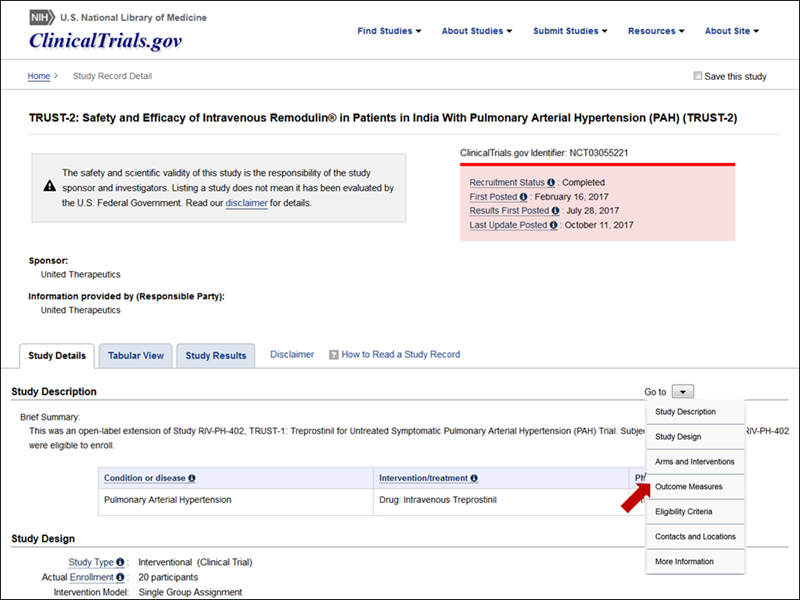
Updated Study Record Layout
The design and layout of the study record page was updated to make the most relevant information more prominent. "Go to" links allow users to access the major sections of a record more easily (see Figure 7).
We welcome your comments, questions, and suggestions. To contact us, please click on "Customer Support" in the footer of the ClinicalTrials.gov site which will take you to the NLM Customer Support page. Then click on "Contact NLM" at the top of the NLM Customer Support page.
By
Karl Wolf, Allison Yu, Nicholas Ide, Rebecca J. Williams, Tony Tse
National Center for Biotechnology Information