



Table of Contents: 2017 SEPTEMBER–OCTOBER No. 418
Wolf K, Ide N, Koufopoulos J. ClinicalTrials.gov: More Changes to Improve Usability. NLM Tech Bull. 2017 Sep-Oct;(418):e7.
On September 25, 2017, the National Library of Medicine (NLM) released updates to ClinicalTrials.gov as the next phase in its ongoing effort to enhance the functionality of the database (see ClinicalTrials.gov: First in a Series of Changes to Improve Usability for Stakeholders). This article highlights key features in the latest release. These changes were informed by user research with end-users representing various stakeholder groups as part of a continuing partnership between NLM and 18F, a federal government digital services consultancy. Additional changes to ClinicalTrials.gov are planned and information about these changes will be provided in future NLM Technical Bulletin notices.
ClinicalTrials.gov is an NLM-maintained resource that provides patients and their families, healthcare professionals, researchers, and members of the public with information about clinical studies and expanded access to investigational drugs (or "compassionate use"). Information listed on ClinicalTrials.gov is provided and updated by the study sponsor or investigator, and listing does not reflect endorsement by the National Institutes of Health. Currently, ClinicalTrials.gov contains information on 255,000 studies and expanded access across the United States and around the world.
Updated Homepage and Disclaimer Text
The homepage has been simplified (see Figure 1). Modified text containing important messages for users, including that listing of a study on ClinicalTrials.gov does not mean the study has been reviewed by the U.S. Federal Government, is displayed on the homepage and at the top of each study record page.
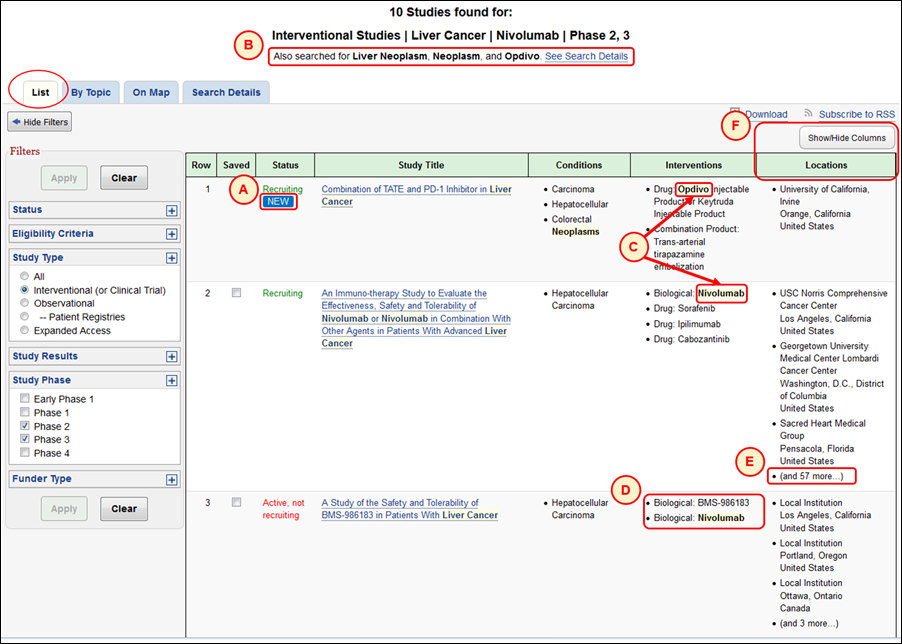
New features on the Search Results page are intended to provide users with additional feedback on what was searched and help users discover study records of interest more quickly. Study records first made available on ClinicalTrials.gov (or "posted") during the past 30 days are identified by the "New" icon in the "Status" column on the List tab (see A in Figure 2). Synonyms of terms used by the search engine are summarized at the top (see B in Figure 2, which shows synonyms for "liver cancer" and "nivolumab") and both search terms and synonyms appearing on the Search Results page are highlighted (see C in Figure 2). Up to three entries can be displayed as a bulleted list in a column (see D in Figure 2); the number of any additional entries is indicated in a fourth bullet (see E in Figure 2). The "Locations" column for displaying study facility information is a new option in the "Show/Hide Columns" panel on the Search Results page (see F in Figure 2).
The Last Updated field was renamed to "Last Update Submitted" and a new field titled "Last Update Posted" is now available. "Last Updated" was defined as the most recent date on which changes to a study record were submitted to ClinicalTrials.gov. That is, the date on which the most recent updates to a study record were made by the study sponsor or investigator and provided to ClinicalTrials.gov. However, delays sometimes occur between the "Last Updated" date (submitted date) and the date on which that updated study information is first accessible on ClinicalTrials.gov. The new Last Update Posted field is defined as the most recent date on which changes to a study record were posted on ClinicalTrials.gov - that is, when updated study information is publicly available.
The Last Update Posted field is displayed on ClinicalTrials.gov study records and appears in the following key features:
Note: Saved searches and RSS feeds created before September 25, 2017, will continue to function, but will use the new Last Update Posted field rather than "Last Updated" (now named "Last Update Submitted"). As explained above, this change may affect the results of a saved search and RSS feed because delays sometimes occur between the time study information is submitted to ClinicalTrials.gov and when it is available to the public.
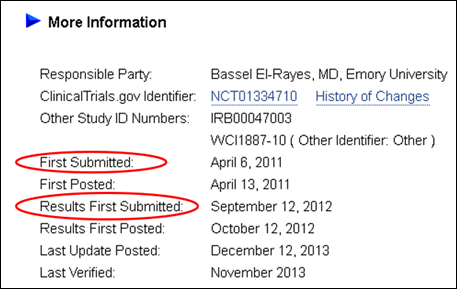
The "First Received" and "Results First Received" date fields have been renamed "First Submitted" and "Results First Submitted," respectively (see Figure 3). Their definitions remain the same (see Glossary of Common Site Terms). The name changes are intended to clarify that these dates indicate when study information was first submitted to ClinicalTrials.gov, not when it was first "posted."
Additional enhancements to ClinicalTrials.gov are still under development and include, but are not limited to:
We welcome your comments, questions, and suggestions. To contact us, please click on "Customer Support" in the footer of the ClinicalTrials.gov Web site which will take you to the NLM Customer Support page. Then click on Contact NLM at the top of the NLM Customer Support page.
By
Karl Wolf, Nicholas Ide, Justin Koufopoulos
National Center for Biotechnology Information